Department of Macromolecular Science, Osaka University* Tokyo University of Agriculture and Technology**
○Kenji Okuyama* Chizuru Hongo* Keiichi Noguchi**
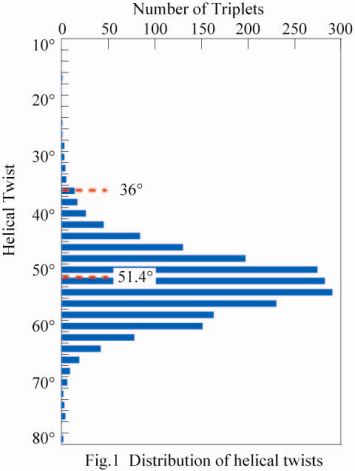
During the last five decades, the 10/3-helical model proposed by Rich and Crick was believed to be the molecular structure of collagen. Although we proposed the different structural model (7/2-helix) based on the single crystal structure of collagen-model peptide (1977), this was only accepted as the structure of model peptides, but not accepted as the structural model for collagen. However, we recently demonstrated that both the 7/2- and 10/3-helical models can explain quantitatively the X-ray diffraction data from native collagen. In this study, we investigated helical twists of 2,163 triplets of the collagen-model peptides found in single crystal structures analyzed at high resolutions. The histogram of helical twists (Fig.1) shows that the center of the distribution (52.6°) agrees very well with the helical twist (51.4°) of the ideal 7/2-helix, while there is no indication of even a very small peak around 36° which corresponds to the ideal twist angle for the 10/3-helical model. These evidences strongly indicated a preference for the 7/2-helical conformation rather than the now prevailing Rich & Crick (10/3-helical) model.
