00066
X-ray Crystal Structure Analysis of a Collagen Model Peptide (Pro-Hyp-Gly)3-Pro-Arg-Gly-(Pro-Hyp-Gly)4
Department of Macromolecular Science, School of Science, Osaka University* Department of Biotechnology and Life Science, Graduate School of Engineering, Tokyo University of Agriculture & Technology, Japan** Department of Macromolecular Science, Graduate School of Science, Osaka University, Japan*** Instrumentation Analysis Center, Tokyo University of Agriculture & Technology, Japan**** Department of Materials Science and Engineering, Graduate School of Engineering, Nagoya Insttitute of technology, Japan*****
○Tatsuya Morimoto* Mitsuru Haga** Chizuru Hongo*** Kenji Okuyama*** Keiichi Noguchi**** Toshiki Tanaka*****
The amino acid sequence of collagen is designated by the repetition of Gly-X-Y triplet. It is well known that Hyp in the Y position stabilizes the triple-helical structure of collagen. Recently, Brodsky and co-workers reported that the host guest peptide with Gly-Pro-Arg sequence as a guest showed a similar helix-coil transition temperature compared with that of Gly-Pro-Hyp sequence. In order to investigate stabilization mechanism of the Gly-Pro-Arg sequence, the host-guest peptide, (Pro-Hyp-Gly)3-Pro-Arg-Gly-(Pro-Hyp-Gly)4 (hereafter PRG), was synthesized and its crystal structure was analyzed at 1.45 Å resolution by using synchrotron radiation (SPring-8).
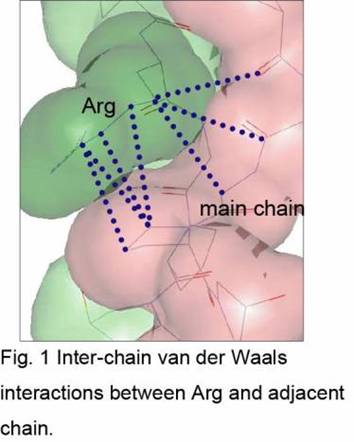
No substantial difference in the main chain conformation was observed between triple-helical structures of the PRG peptide and previously analyzed (Pro-Hyp-Gly)11. In the triple-helix of the PRG peptide, the side chain of Arg seems to stabilize its structure in two ways. One is van der Waals interactions between the long side chain of Arg and several parts of the adjacent chain (Fig1). The other is water-mediated hydrogen bonds between one of three nitrogen atoms in the Arg side chain and one of oxygen atoms in the same chain or in the adjacent chain.