Department of Medical Biochemistry, Kurume University School of Medicine* Department of Biology, Graduate School of Science, Osaka University, Japan**
○Masakazu Sugishima* Yoshinori Hagiwara** Yasuhiro Takahashi** Keiichi Fukuyama**
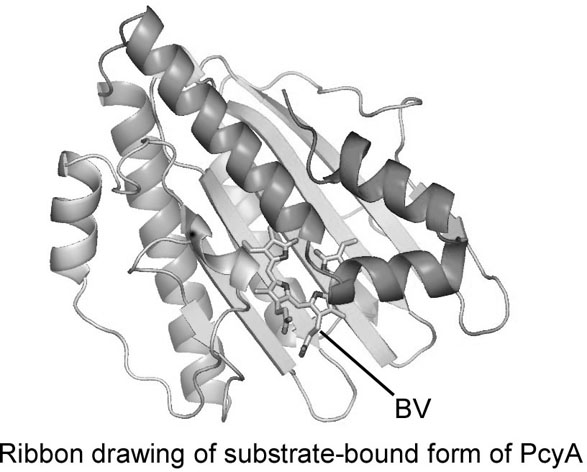
Phytobilins are linear tetrapyrrole compounds used as pigments for light-harvesting (phycobiliproteins) and photoreceptor (phytochromes) proteins in O2 producing photosynthetic organisms, such as cyanobacteria and plants. Phytobilins are biosynthesized from biliverdin (BV), a catabolite of heme, by ferredoxin-dependent bilin reductases (FDBRs). Phycocyanobilin:ferredoxin oxidoreductase (PcyA) one such FDBR, is a new class of radical enzymes that require neither cofactors nor metals, and serially reduces the vinyl group of the D-ring and A-ring of BV using four-electrons from ferredoxin to produce phycocyanobilin. We have determined the crystal structure of substrate-bound and substrate-free forms of cyanobacterial PcyA, the first tertiary structure in FDBRs. PcyA folds into three-layer alpha/beta/alpha sandwich and BV is located between the beta-sheet and C-terminal alpha-helices. The structure of substrate-free form of PcyA is similar to that of substrate-bound form, but the charge distribution and the active site structure slightly differ. Recognition of substrate and ferredoxin and reaction mechanism to control the serial reductions of the D- and A-rings of BV will be presented.
