Department of Physics, Yokohama National University
○Kazuyuki Sato Kazuyuki Hino Hiroo Takahashi Jin Mizuguchi
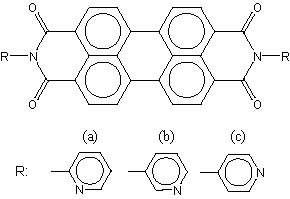
We have recently developed a H2 gas sensor utilizing a high proton affinity of diketodipyridylpyrrolopyrrole (para-DPPP). Protonation is found to bring about remarkable reduction of the electrical resistivity and this effect has been applied to H2 gas sensors. In the present investigation, we extend our idea to a larger chromophore such as a perylene-imide skeleton in order to improve the sensor characteristics. We have synthesized novel perylene-imide derivatives connected directly with proton accepting pyridyl rings. The two N atoms of the pyridyl rings which work as proton acceptors are found to remain unbonded (i.e. free) in the solid state according to X-ray analysis and are thus available for protonation necessary for H2 gas sensors. The present sensors exhibit a significant change in electrical resistivity by about three orders of magnitude even under 0.05 % H2 and a higher sensitivity by one order of magnitude as compared with that of p-DPPP. Furthermore, these sensors are found to operate reversibly at room temperature and are scarcely influenced by ambient gases such as CH4, CO, CO2, NO, SO2, CH3OH, C2H5OH and H2O moisture.
