(1) T. Kubota et al. J. Mol. Biol., 359, 708-727 (2006)
Structural Biology Research Center, PF, IMSS, KEK, Present address: Graduate School of Arts and Sciences, The University of Tokyo* Glycogene Function Team of Research Center for Glycoscience (RCG), AIST, Japan** Structural Biology Research Center, PF, IMSS, KEK, Japan***
○Tomoo Shiba* Tomomi Kubota** Shigemi Sugioka** Sanae Furukawa** Hiromichi Sawaki** Ryuich Kato*** Soichi Wakatsuki*** Hisashi Narimatsu**
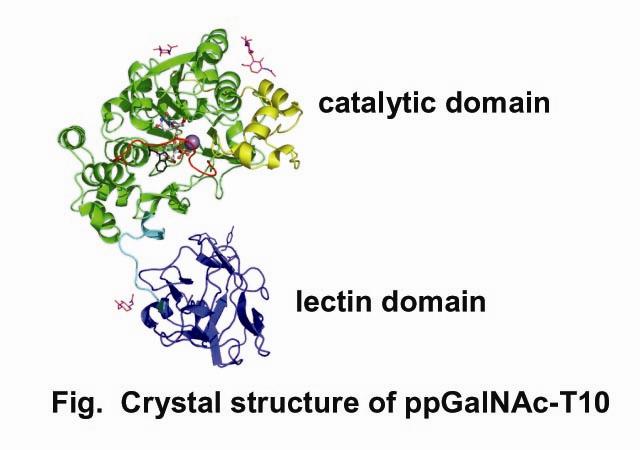
Biosynthesis of mucin-type O-glycan is initiated by the transfer of GalNAc which is catalyzed by UDP-GalNAc:polypeptide α-N-acetylgalactosaminyltransferases (pp-GalNAc-Ts). To date, 15 human isozymes have been reported. Here we present crystal structures of the pp-GalNAc-T10 isozyme, which has specificity for glycosylated peptides, in complex with the hydrolyzed donor substrate UDP-GalNAc and in complex with GalNAc-serine (1). pp-GalNAc-T10 comprises two domains, catalytic and lectin domains (Fig), similarly to pp-GalNAc-T1 and pp-GalNAc-T2, which are reported by Fritz et al [2004 and 2006]. However, a distinct interdomain arrangement of pp-GalNAc-T10 results in a narrow cleft for acceptor substrates, compared with pp-GalNAc-T1 and pp-GalNAc-T2. The two domains are connected through a linker region, whose amino-acid sequences are not conserved among pp-GalNAc-Ts. GalNAc-Ser is bound to only the lectin β subdomain but not to the other two subdomains. The distance between the catalytic center and the carbohydrate-binding site on the lectin β subdomain influences the position of GalNAc glycosylation on GalNAc-glycosylated peptide substrates.
(1) T. Kubota et al. J. Mol. Biol., 359, 708-727 (2006)
