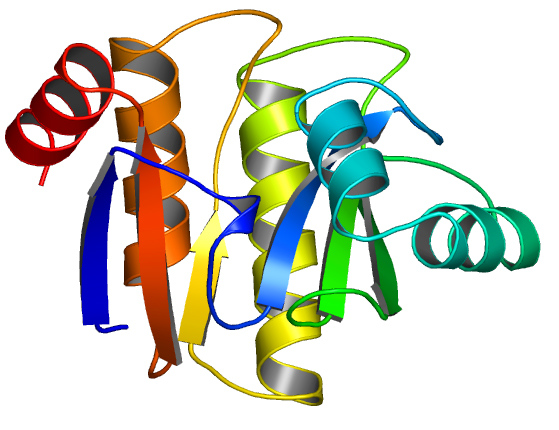Reference
[1] Karras, G.I., Kustatscher, G., Buhecha, H.R., Allen, M.D., Pugieux, C., Sait, F., Bycroft, M. & Ladurner, A.G. Embo J 24, 1911-20 (2005).
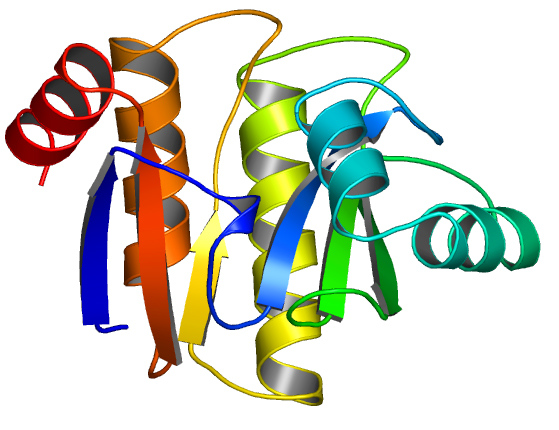
RIKEN GSC* Tohoku Univ.** RIKEN Harima***
○Seiichiro Kishishita* Kazutaka Murayama** Takaho Terada* Mikako Shirouzu* Seiki Kuramitsu*** Shigeyuki Yokoyama*
We solved the crystal structure of a hypothetical protein, TTHA0132 from Thermus thermophilus HB8 at 1.77Å resolution. TTHA0132 indicates 35% homology against macro-domain on histone protein, macroH2A. The structure of TTHA0132 has a mixed alpha/beta fold that closely resembles the N-terminal DNA binding domain of the Escherichia coli leucine aminopeptidase, PepA. Karras et al. reported that the macro-domain contains ADP-ribose binding site [1]. In the crystal structure of TTHA0132, this binding site for ADP-ribose is highly conserved. These results suggest that the TTHA0132 plays an important role on ADP-ribosylation. We will discuss about the functions of TTHA0132 based on the structure.
Reference
[1] Karras, G.I., Kustatscher, G., Buhecha, H.R., Allen, M.D., Pugieux, C., Sait, F., Bycroft, M. & Ladurner, A.G. Embo J 24, 1911-20 (2005).