00353
Vapor induced solid-state phase transition and photoreactivity change investigated by ab initio powder crystal structure determination
Department of Chemistry and Materials Science, Tokyo Institute of Technology* Department of Chemistry, Okayama University of Science, JAPAN**
○Kotaro Fujii* Yasunari Ashida* Hidehiro Uekusa* Hirano Shinya** Shinji Toyota** Fumio Toda**
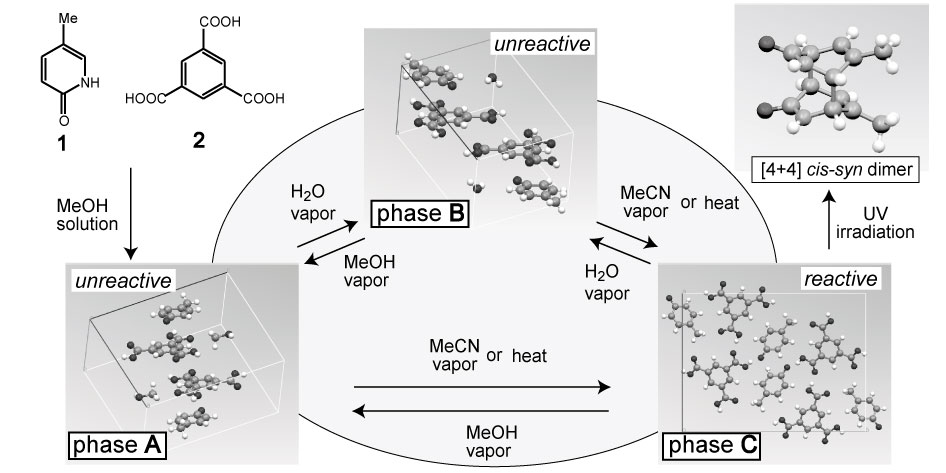
Inclusion complex crystal of 5-methyl-2-pyridone (1) and trimesic acid (2), exhibits variety of solid-state phase transitions induced by solvent vapor contact. Thus, modification of the photoreactivity of pyridone molecules in crystal is expected. A 1:1:1 inclusion complex of 1, 2 and MeOH (phase A; unreactive) was transformed to the unreactive phase B by water vapor contact. But both A and B were transformed by MeCN vapor contact or by heating to photoreactive phase C and [4+4] cis-syn dimer molecule was obtained by UV irradiation. The structure of B and C were successfully determined by ab initio powder crystal structure analysis in spite of the disintegration to powder crystalline state during phase transition.
Phase B is formed after crystalline solvent exchange of MeOH for H2O and the structure resembles to A. Both are unreactive due to lack of suitable pyridone molecules arrangement. Phase C has a reactive distance (3.84 Å) and an arrangement to produce cis-syn dimer. This arrangement was achieved by turning of a pyridone molecule about 180° during removal of the solvent molecule.