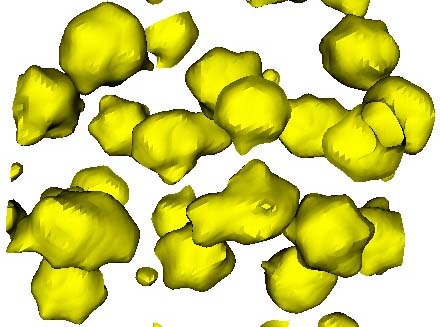
Figure. Electron density distribution of tetradecameric water cluster in [(C6H5)4P]3 [H3V10O28].7H2O.
Department of Chemistry and Materials Science, Tokyo Institute of Technology* Quantum Beam Science Directorate, Japan Atomic Energy Agency**
○Tomoji Ozeki* Setsuko Nakamura* Takuto Yamawaki* Katsuhiro Kusaka**
Many compounds crystallize as hydrated forms when precipitated from aqueous or water-containing solutions. Water plays an important role in constructing such crystals. One extreme example of them is the clathrates of hydrophobic gaseous molecules. Another extreme example may be water clusters enclathrated in hydrophobic environments. We have synthesized discrete water clusters enclathrated in hydrophobic channels composed by the tetraphenylphosphonium cations, which may be regarded as examples of water clusters in hydrophobic environments. The tetraphenylphosphonium cations self-assemble by the C-H...p interactions to form three-dimensional networks, between which polyoxometalate anions are incorporated. However, the sizes and charges of the cations and anions are incompatible and thus void spaces remain unoccupied. These voids lead to the formation of discrete water clusters. In one of these examples, the distribution of the clusters become ordered or disordered depending on the crystallization conditions, resulting in the disappearance/appearance of the diffuse scattering recorded on its single crystal diffraction images.
Figure. Electron density distribution of tetradecameric water cluster in [(C6H5)4P]3 [H3V10O28].7H2O.
