Department of Life Science, University of Hyogo and RIKEN Harima Inst.* Okazaki Inst.for Integrative Biosci.**
○Hirofumi Komori* Sayaka Inagaki** Shiro Yoshioka** Shigetoshi Aono** Yoshiki Higuchi*
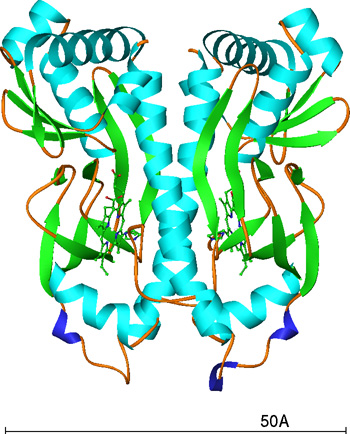
CooA, homodimeric heme-containning protein, is responsible for the transcriptional regulation in response to carbon monoxide (CO). Only CO-bound CooA can bind to the target DNA to be active as a transcription activator. CO replaces the N-terminus coordinated to the heme iron in the inactive form upon CO binding, which induces a conformational change to activate CooA. Although this ligand exchange is thought to be a trigger of the activation of CooA by CO, the detailed mechanism is unknown. Here, we have determined the crystal structure of the CooA from Carboxydothermus hydrogenoformans (Ch-CooA). Ch-CooA was overexpressed in Escherichia coli, purified and crystallized hanging-drop vapour-diffusion method. The crystal diffracted to a resolution of 2.3 A resolution. They are monoclinic and belong to space group P21, with unit-cell parameters a = 61.8, b = 94.7, c = 92.8 and beta = 104.8. The structure was solved by the single wavelength anomalous diffraction (SAD) method. We report the structural features of the Ch-CooA and the CO-sensing mechanism.
